Emergency Standby Power Systems Pdf Writer
Scheme of a proton-conducting fuel cell A fuel cell is an that converts the from a fuel into electricity through an reaction of with oxygen or another. Fuel cells are different from in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy comes from chemicals already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
The first fuel cells were invented in 1838. The first commercial use of fuel cells came more than a century later in space programs to generate power for and. Since then, fuel cells have been used in many other applications.
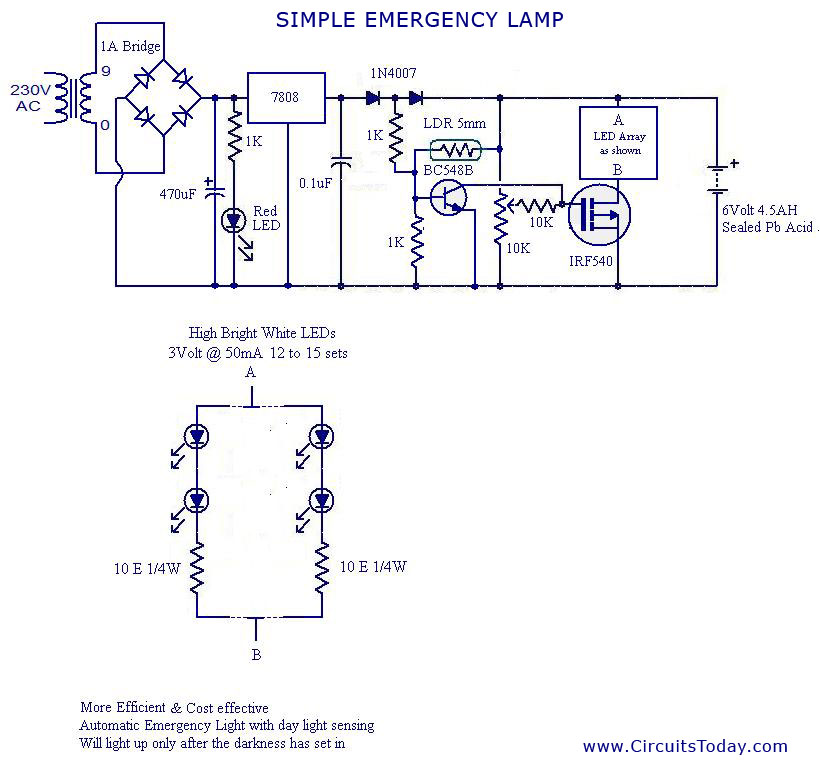
Sep 14, 2014. Emergency Power Systems for Critical Facilities: A Best Practices Approach to. Improving Reliability. APPLIED TECHNOLOGY COUNCIL. 201 Redwood Shores Parkway, Suite 240. Redwood City, California 94065 www.ATCouncil.org. Prepared for. FEDERAL EMERGENCY MANAGEMENT. Jan 11, 2013. NEC Requirements for Generators and Standby Power Systems. Transfer equipment must supply only emergency loads. Figure 700–4. Author's Comment: Multiple transfer switches are required where a single generator is used to supply both emergency loads and other loads.
Fuel cells are used for primary and backup power for commercial, industrial and residential buildings and in remote or inaccessible areas. They are also used to power, including forklifts, automobiles, buses, boats, motorcycles and submarines. There are many types of fuel cells, but they all consist of an, a, and an that allows positively charged hydrogen ions (protons) to move between the two sides of the fuel cell. At the anode a catalyst causes the fuel to undergo oxidation reactions that generate protons (positively charged hydrogen ions) and electrons.
The protons flow from the anode to the cathode through the electrolyte after the reaction. At the same time, electrons are drawn from the anode to the cathode through an external circuit, producing electricity. At the cathode, another catalyst causes hydrogen ions, electrons, and oxygen to react, forming water.
Fuel cells are classified by the type of electrolyte they use and by the difference in startup time ranging from 1 second for (PEM fuel cells, or PEMFC) to 10 minutes for (SOFC). Individual fuel cells produce relatively small electrical potentials, about 0.7 volts, so cells are 'stacked', or placed in series, to create sufficient voltage to meet an application's requirements.
In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, very small amounts of and other emissions. The energy efficiency of a fuel cell is generally between 40–60%; however, if waste heat is captured in a scheme, efficiencies up to 85% can be obtained. A related technology is, in which the fuel can be regenerated by recharging. The fuel cell market is growing, and in 2013 Pike Research estimated that the stationary fuel cell market will reach 50 GW by 2020. Sketch of William Grove's 1839 fuel cell The first references to hydrogen fuel cells appeared in 1838. In a letter dated October 1838 but published in the December 1838 edition of The London and Edinburgh Philosophical Magazine and Journal of Science, Welsh physicist and barrister wrote about the development of his first crude fuel cells.
He used a combination of sheet iron, copper and porcelain plates, and a solution of sulphate of copper and dilute acid. In a letter to the same publication written in December 1838 but published in June 1839, German physicist discussed the first crude fuel cell that he had invented.
His letter discussed current generated from hydrogen and oxygen dissolved in water. Grove later sketched his design, in 1842, in the same journal. The fuel cell he made used similar materials to today's. In 1939, British engineer successfully developed a 5 kW stationary fuel cell. Thomas Grubb, a chemist working for the Company (GE), further modified the original fuel cell design by using a sulphonated polystyrene ion-exchange membrane as the electrolyte. Three years later another GE chemist, Leonard Niedrach, devised a way of depositing platinum onto the membrane, which served as catalyst for the necessary hydrogen oxidation and oxygen reduction reactions.
This became known as the 'Grubb-Niedrach fuel cell'. GE went on to develop this technology with NASA and McDonnell Aircraft, leading to its use during. This was the first commercial use of a fuel cell. In 1959, a team led by Harry Ihrig built a 15 kW fuel cell tractor for, which was demonstrated across the U.S. At state fairs. This system used potassium hydroxide as the electrolyte and and oxygen as the reactants.
Later in 1959, Bacon and his colleagues demonstrated a practical five-kilowatt unit capable of powering a welding machine. In the 1960s, Pratt and Whitney licensed Bacon's U.S. Patents for use in the U.S.
Space program to supply electricity and drinking water (hydrogen and oxygen being readily available from the spacecraft tanks). In 1991, the first hydrogen fuel cell automobile was developed. Was the first company to manufacture and commercialize a large, stationary fuel cell system for use as a power plant in hospitals, universities and large office buildings. In recognition of the fuel cell industry and America’s role in fuel cell development, the US Senate recognized 8 October 2015 as National Hydrogen and Fuel Cell Day, passing S. The date was chosen in recognition of the atomic weight of hydrogen (1.008). Types of fuel cells; design [ ] Fuel cells come in many varieties; however, they all work in the same general manner. They are made up of three adjacent segments: the, the, and the.
Two chemical reactions occur at the interfaces of the three different segments. The net result of the two reactions is that fuel is consumed, water or carbon dioxide is created, and an electric current is created, which can be used to power electrical devices, normally referred to as the load. At the anode a oxidizes the fuel, usually hydrogen, turning the fuel into a positively charged ion and a negatively charged electron. The electrolyte is a substance specifically designed so ions can pass through it, but the electrons cannot. The freed electrons travel through a wire creating the electric current.
The ions travel through the electrolyte to the cathode. Once reaching the cathode, the ions are reunited with the electrons and the two react with a third chemical, usually oxygen, to create water or carbon dioxide. A block diagram of a fuel cell The most important design features in a fuel cell are [ ]: • The electrolyte substance.
The electrolyte substance usually defines the type of fuel cell. • The fuel that is used. The most common fuel is hydrogen.
• The anode catalyst breaks down the fuel into electrons and ions. The anode catalyst is usually made up of very fine platinum powder. • The cathode catalyst turns the ions into the products like water or carbon dioxide. The cathode catalyst is often made up of platinum or platinum-group metals and it can also be made of non-platinum metals such as iron. A typical fuel cell produces a voltage from 0.6 V to 0.7 V at full rated load. Voltage decreases as current increases, due to several factors: • • Ohmic loss ( due to resistance of the cell components and interconnections) • Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage). To deliver the desired amount of energy, the fuel cells can be combined in to yield higher, and in parallel to allow a higher to be supplied.
Such a design is called a fuel cell stack. The cell surface area can also be increased, to allow higher current from each cell.
Within the stack, reactant gases must be distributed uniformly over each of the cells to maximize the power output. Proton exchange membrane fuel cells (PEMFCs) [ ]. Main article: In the archetypical hydrogen–oxide design, a proton-conducting polymer membrane (typically ) contains the solution that separates the and sides. This was called a 'solid polymer electrolyte fuel cell' (SPEFC) in the early 1970s, before the proton exchange mechanism was well understood. (Notice that the synonyms 'polymer electrolyte membrane' and 'proton exchange mechanism' result in the same.) On the anode side, hydrogen diffuses to the anode catalyst where it later dissociates into protons and electrons. These protons often react with oxidants causing them to become what are commonly referred to as multi-facilitated proton membranes. The protons are conducted through the membrane to the cathode, but the electrons are forced to travel in an external circuit (supplying power) because the membrane is electrically insulating.
On the cathode catalyst, oxygen react with the electrons (which have traveled through the external circuit) and protons to form water. In addition to this pure hydrogen type, there are fuels for fuel cells, including, ( see: and ) and chemical hydrides.
The waste products with these types of fuel are and water. When hydrogen is used, the CO2 is released when methane from natural gas is combined with steam, in a process called, to produce the hydrogen. This can take place in a different location to the fuel cell, potentially allowing the hydrogen fuel cell to be used indoors—for example, in fork lifts. Condensation of water produced by a PEMFC on the air channel wall. The gold wire around the cell ensures the collection of electric current.
The different components of a PEMFC are • bipolar plates, •, •, • membrane, and • the necessary hardware such as current collectors and gaskets. The materials used for different parts of the fuel cells differ by type. The bipolar plates may be made of different types of materials, such as, metal, coated metal,, flexible graphite, C–C, – composites etc. The (MEA) is referred as the heart of the PEMFC and is usually made of a proton exchange membrane sandwiched between two -coated. Platinum and/or similar type of are usually used as the catalyst for PEMFC. The electrolyte could be a polymer.
Proton exchange membrane fuel cell design issues [ ] • Cost. In 2013, the Department of Energy estimated that 80-kW automotive fuel cell system costs of US$67 per kilowatt could be achieved, assuming volume production of 100,000 automotive units per year and US$55 per kilowatt could be achieved, assuming volume production of 500,000 units per year.
Many companies are working on techniques to reduce cost in a variety of ways including reducing the amount of platinum needed in each individual cell. Has experimented with a catalyst enhanced with carbon silk, which allows a 30% reduction (1 mg/cm² to 0.7 mg/cm²) in platinum usage without reduction in performance., uses as a. A 2011 published study doi: 10.1021/ja1112904 documented the first metal-free electrocatalyst using relatively inexpensive doped, which are less than 1% the cost of platinum and are of equal or superior performance.
A recently published article demonstrated how the environmental burdens change when using carbon nanotubes as carbon substrate for platinum. • Water and air management (in PEMFCs). In this type of fuel cell, the membrane must be hydrated, requiring water to be evaporated at precisely the same rate that it is produced. If water is evaporated too quickly, the membrane dries, resistance across it increases, and eventually it will crack, creating a gas 'short circuit' where hydrogen and oxygen combine directly, generating heat that will damage the fuel cell. If the water is evaporated too slowly, the electrodes will flood, preventing the reactants from reaching the catalyst and stopping the reaction. Methods to manage water in cells are being developed like focusing on flow control. Just as in a combustion engine, a steady ratio between the reactant and oxygen is necessary to keep the fuel cell operating efficiently.
• Temperature management. The same temperature must be maintained throughout the cell in order to prevent destruction of the cell through. This is particularly challenging as the 2H 2 + O 2 ->2H 2O reaction is highly exothermic, so a large quantity of heat is generated within the fuel cell. • Durability,, and special requirements for some type of cells.
Typically require more than 40,000 hours of reliable operation at a temperature of −35 °C to 40 °C (−31 °F to 104 °F), while automotive fuel cells require a 5,000-hour lifespan (the equivalent of 240,000 km (150,000 mi)) under extreme temperatures. Current is 2,500 hours (about 75,000 miles). Automotive engines must also be able to start reliably at −30 °C (−22 °F) and have a high power-to-volume ratio (typically 2.5 kW per liter). • Limited tolerance of some (non-PEDOT) cathodes.
Phosphoric acid fuel cell (PAFC) [ ]. Main article: Phosphoric acid fuel cells (PAFC) were first designed and introduced in 1961 by and. In these cells phosphoric acid is used as a non-conductive electrolyte to pass positive hydrogen ions from the anode to the cathode. These cells commonly work in temperatures of 150 to 200 degrees Celsius. This high temperature will cause heat and energy loss if the heat is not removed and used properly.
This heat can be used to produce steam for air conditioning systems or any other thermal energy consuming system. Using this heat in can enhance the efficiency of phosphoric acid fuel cells from 40–50% to about 80%. Phosphoric acid, the electrolyte used in PAFCs, is a non-conductive liquid acid which forces electrons to travel from anode to cathode through an external electrical circuit. Since the hydrogen ion production rate on the anode is small, platinum is used as catalyst to increase this ionization rate. A key disadvantage of these cells is the use of an acidic electrolyte. This increases the corrosion or oxidation of components exposed to phosphoric acid.
Solid acid fuel cell (SAFC) [ ]. Main article: Solid acid fuel cells (SAFCs) are characterized by the use of a solid acid material as the electrolyte. At low temperatures, have an ordered molecular structure like most salts. At warmer temperatures (between 140 and 150 degrees Celsius for CsHSO 4), some solid acids undergo a phase transition to become highly disordered 'superprotonic' structures, which increases conductivity by several orders of magnitude. The first proof-of-concept SAFCs were developed in 2000 using cesium hydrogen sulfate (CsHSO 4). Current SAFC systems use cesium dihydrogen phosphate (CsH 2PO 4) and have demonstrated lifetimes in the thousands of hours.
Alkaline fuel cell (AFC) [ ]. Main articles: and The alkaline fuel cell or hydrogen-oxygen fuel cell was designed and first demonstrated publicly by Francis Thomas Bacon in 1959. It was used as a primary source of electrical energy in the Apollo space program.
The cell consists of two porous carbon electrodes impregnated with a suitable catalyst such as Pt, Ag, CoO, etc. The space between the two electrodes is filled with a concentrated solution of KOH or NaOH which serves as an electrolyte.
H 2 gas and O 2 gas are bubbled into the electrolyte through the porous carbon electrodes. Thus the overall reaction involves the combination of hydrogen gas and oxygen gas to form water. The cell runs continuously until the reactant's supply is exhausted. This type of cell operates efficiently in the temperature range 343 K to 413 K and provides a potential of about 0.9 V. Is a type of AFC which employs a solid polymer electrolyte instead of aqueous potassium hydroxide (KOH) and it is superior to aqueous AFC.
High-temperature fuel cells [ ] SOFC [ ]. Main article: (SOFCs) use a solid material, most commonly a ceramic material called (YSZ), as the. Because SOFCs are made entirely of solid materials, they are not limited to the flat plane configuration of other types of fuel cells and are often designed as rolled tubes. They require high (800–1000 °C) and can be run on a variety of fuels including natural gas.
SOFCs are unique since in those, negatively charged oxygen travel from the (positive side of the fuel cell) to the (negative side of the fuel cell) instead of positively charged hydrogen ions travelling from the anode to the cathode, as is the case in all other types of fuel cells. Oxygen gas is fed through the cathode, where it absorbs electrons to create oxygen ions. The oxygen ions then travel through the electrolyte to react with hydrogen gas at the anode. The reaction at the anode produces electricity and water as by-products. Carbon dioxide may also be a by-product depending on the fuel, but the carbon emissions from an SOFC system are less than those from a fossil fuel combustion plant.
The chemical reactions for the SOFC system can be expressed as follows: Anode Reaction: 2H 2 + 2O 2− → 2H 2O + 4e − Cathode Reaction: O 2 + 4e − → 2O 2− Overall Cell Reaction: 2H 2 + O 2 → 2H 2O SOFC systems can run on fuels other than pure hydrogen gas. However, since hydrogen is necessary for the reactions listed above, the fuel selected must contain hydrogen atoms. For the fuel cell to operate, the fuel must be converted into pure hydrogen gas.
SOFCs are capable of internally light hydrocarbons such as (natural gas), propane and butane. These fuel cells are at an early stage of development. Challenges exist in SOFC systems due to their high operating temperatures.
One such challenge is the potential for carbon dust to build up on the anode, which slows down the internal reforming process. Research to address this 'carbon coking' issue at the University of Pennsylvania has shown that the use of copper-based (heat-resistant materials made of ceramic and metal) can reduce coking and the loss of performance. Another disadvantage of SOFC systems is slow start-up time, making SOFCs less useful for mobile applications. Despite these disadvantages, a high operating temperature provides an advantage by removing the need for a precious metal catalyst like platinum, thereby reducing cost. Additionally, waste heat from SOFC systems may be captured and reused, increasing the theoretical overall efficiency to as high as 80%–85%. The high operating temperature is largely due to the physical properties of the YSZ electrolyte. As temperature decreases, so does the of YSZ.
Therefore, to obtain optimum performance of the fuel cell, a high operating temperature is required. According to their website,, a UK SOFC fuel cell manufacturer, has developed a method of reducing the operating temperature of their SOFC system to 500–600 degrees Celsius. They replaced the commonly used YSZ electrolyte with a CGO (cerium gadolinium oxide) electrolyte. The lower operating temperature allows them to use stainless steel instead of ceramic as the cell substrate, which reduces cost and start-up time of the system.
Main article: (MCFCs) require a high operating temperature, 650 °C (1,200 °F), similar to. MCFCs use lithium potassium carbonate salt as an electrolyte, and this salt liquefies at high temperatures, allowing for the movement of charge within the cell – in this case, negative carbonate ions.
Like SOFCs, MCFCs are capable of converting fossil fuel to a hydrogen-rich gas in the anode, eliminating the need to produce hydrogen externally. The reforming process creates CO 2 emissions. MCFC-compatible fuels include natural gas, biogas and gas produced from coal.
The hydrogen in the gas reacts with carbonate ions from the electrolyte to produce water, carbon dioxide, electrons and small amounts of other chemicals. The electrons travel through an external circuit creating electricity and return to the cathode.
There, oxygen from the air and carbon dioxide recycled from the anode react with the electrons to form carbonate ions that replenish the electrolyte, completing the circuit. The chemical reactions for an MCFC system can be expressed as follows: Anode Reaction: CO 3 2− + H 2 → H 2O + CO 2 + 2e − Cathode Reaction: CO 2 + ½O 2 + 2e − → CO 3 2− Overall Cell Reaction: H 2 + ½O 2 → H 2O As with SOFCs, MCFC disadvantages include slow start-up times because of their high operating temperature.
This makes MCFC systems not suitable for mobile applications, and this technology will most likely be used for stationary fuel cell purposes. The main challenge of MCFC technology is the cells' short life span. The high-temperature and carbonate electrolyte lead to corrosion of the anode and cathode. These factors accelerate the degradation of MCFC components, decreasing the durability and cell life. Researchers are addressing this problem by exploring corrosion-resistant materials for components as well as fuel cell designs that may increase cell life without decreasing performance.
MCFCs hold several advantages over other fuel cell technologies, including their resistance to impurities. They are not prone to 'carbon coking', which refers to carbon build-up on the anode that results in reduced performance by slowing down the internal fuel process.
Therefore, carbon-rich fuels like gases made from coal are compatible with the system. The Department of Energy claims that coal, itself, might even be a fuel option in the future, assuming the system can be made resistant to impurities such as sulfur and particulates that result from converting coal into hydrogen.
MCFCs also have relatively high efficiencies. They can reach a fuel-to-electricity efficiency of 50%, considerably higher than the 37–42% efficiency of a phosphoric acid fuel cell plant.
Efficiencies can be as high as 65% when the fuel cell is paired with a turbine, and 85% if heat is captured and used in a (CHP) system. FuelCell Energy, a Connecticut-based fuel cell manufacturer, develops and sells MCFC fuel cells. The company says that their MCFC products range from 300 kW to 2.8 MW systems that achieve 47% electrical efficiency and can utilize CHP technology to obtain higher overall efficiencies. One product, the DFC-ERG, is combined with a gas turbine and, according to the company, it achieves an electrical efficiency of 65%. Electric storage fuel cell [ ] The electric storage fuel cell is a conventional battery chargeable by electric power input, using the conventional electro-chemical effect. However, the battery further includes hydrogen (and oxygen) inputs for alternatively charging the battery chemically.
Comparison of fuel cell types [ ] Fuel cell name Electrolyte Qualified (W) Working temperature (°C) (cell) Efficiency (system) Status Cost (USD/W) solution 0! >-20 (50% P peak @ 0 °C) Aqueous alkaline solution 39!
90–120 Research Polymer membrane (ionomer) 100! 1 W – 500 kW 125! 50–100 (Nafion) 120–200 (PBI) 60%! 30–50% Commercial / Research 50–100 Liquid electrolytes with shuttle and polymer membrane (Ionomer) 1000!
1 kW – 10 MW Research Molten (H 3PO 4) 999999! With fuel cell propulsion of the in dry dock Power [ ] Stationary fuel cells are used for commercial, industrial and residential primary and backup power generation. Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, communications centers, rural locations including research stations, and in certain military applications.
A fuel cell system running on hydrogen can be compact and lightweight, and have no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability. This equates to less than one minute of downtime in a six-year period. Since fuel cell electrolyzer systems do not store fuel in themselves, but rather rely on external storage units, they can be successfully applied in large-scale energy storage, rural areas being one example. There are many different types of stationary fuel cells so efficiencies vary, but most are between 40% and 60% energy efficient. However, when the fuel cell's waste heat is used to heat a building in a cogeneration system this efficiency can increase to 85%.
This is significantly more efficient than traditional coal power plants, which are only about one third energy efficient. Assuming production at scale, fuel cells could save 20–40% on energy costs when used in cogeneration systems. Fuel cells are also much cleaner than traditional power generation; a fuel cell power plant using natural gas as a hydrogen source would create less than one ounce of pollution (other than CO 2) for every 1,000 kWh produced, compared to 25 pounds of pollutants generated by conventional combustion systems. Fuel Cells also produce 97% less nitrogen oxide emissions than conventional coal-fired power plants. One such pilot program is operating on in Washington State.
There the Stuart Island Energy Initiative has built a complete, closed-loop system: Solar panels power an electrolyzer, which makes hydrogen. The hydrogen is stored in a 500-U.S.-gallon (1,900 L) tank at 200 pounds per square inch (1,400 kPa), and runs a ReliOn fuel cell to provide full electric back-up to the off-the-grid residence. Another closed system loop was unveiled in late 2011 in Hempstead, NY.
Fuel cells can be used with low-quality gas from landfills or waste-water treatment plants to generate power and lower. A 2.8 MW fuel cell plant in California is said to be the largest of the type.
Cogeneration [ ] Combined heat and power (CHP) fuel cell systems, including (MicroCHP) systems are used to generate both electricity and heat for homes (see ), office building and factories. The system generates constant electric power (selling excess power back to the grid when it is not consumed), and at the same time produces hot air and water from the. As the result CHP systems have the potential to save primary energy as they can make use of waste heat which is generally rejected by thermal energy conversion systems. A typical capacity range of is 1–3 kW el / 4–8 kW th. CHP systems linked to use their waste heat for. The waste heat from fuel cells can be diverted during the summer directly into the ground providing further cooling while the waste heat during winter can be pumped directly into the building. The University of Minnesota owns the patent rights to this type of system Co-generation systems can reach 85% efficiency (40–60% electric + remainder as thermal).
Phosphoric-acid fuel cells (PAFC) comprise the largest segment of existing CHP products worldwide and can provide combined efficiencies close to 90%. Molten Carbonate (MCFC) and Solid Oxide Fuel Cells (SOFC) are also used for combined heat and power generation and have electrical energy efficiences around 60%. Disadvantages of co-generation systems include slow ramping up and down rates, high cost and short lifetime. Also their need to have a hot water storage tank to smooth out the thermal heat production was a serious disadvantage in the domestic market place where space in domestic properties is at a great premium. Delta-ee consultants stated in 2013 that with 64% of global sales the fuel cell micro-combined heat and power passed the conventional systems in sales in 2012.
The Japanese ENE FARM project will pass 100,000 FC mCHP systems in 2014, 34.213 PEMFC and 2.224 SOFC were installed in the period 2012-2014, 30,000 units on and 6,000 on. Fuel cell electric vehicles (FCEVs) [ ]. Fuel cell vehicle Automobiles [ ] As of 2015, two have been introduced for commercial lease and sale in limited quantities: the and the. Additional demonstration models include the, and.
As of June 2011 demonstration FCEVs had driven more than 4,800,000 km (3,000,000 mi), with more than 27,000 refuelings. Demonstration fuel cell vehicles have been produced with 'a driving range of more than 400 km (250 mi) between refueling'. They can be refueled in less than 5 minutes. Department of Energy's Fuel Cell Technology Program claims that, as of 2011, fuel cells achieved 53–59% efficiency at one-quarter power and 42–53% vehicle efficiency at full power, and a durability of over 120,000 km (75,000 mi) with less than 10% degradation. In a Well-to-Wheels simulation analysis that 'did not address the economics and market constraints', General Motors and its partners estimated that per mile traveled, a fuel cell electric vehicle running on compressed gaseous hydrogen produced from natural gas could use about 40% less energy and emit 45% less greenhouse gasses than an internal combustion vehicle. A lead engineer from the Department of Energy whose team is testing fuel cell cars said in 2011 that the potential appeal is that 'these are full-function vehicles with no limitations on range or refueling rate so they are a direct replacement for any vehicle.
For instance, if you drive a full sized SUV and pull a boat up into the mountains, you can do that with this technology and you can't with current battery-only vehicles, which are more geared toward city driving.' In 2014, Toyota introduced its first fuel cell vehicle in Japan, the Mirai, at a price of less than US$100,000, although former European Parliament President estimates that Toyota will initially lose about $100,000 on each Mirai sold. Hyundai introduced the limited production. Other manufacturers that announced intentions to sell fuel cell electric vehicles commercially by 2016 included General Motors, Honda, Mercedes-Benz, and Nissan, but by 2017, most of the automobile companies developing hydrogen cars had switched their focus to battery electric vehicles. Criticism [ ] Some experts believe that hydrogen fuel cell cars will never become economically competitive with other technologies or that it will take decades for them to become profitable. Elon Musk stated in 2015 that fuel cells for use in cars will never be commercially viable because of the inefficiency of producing, transporting and storing hydrogen and the flammability of the gas, among other reasons. Professor Jeremy P.
Meyers estimated in 2008 that cost reductions over a production ramp-up period will take about 20 years after fuel-cell cars are introduced before they will be able to compete commercially with current market technologies, including gasoline internal combustion engines. In 2011, the chairman and CEO of,, stated that while the cost of hydrogen fuel cell cars is decreasing: 'The car is still too expensive and probably won't be practical until the 2020-plus period, I don't know.' In 2012, Lux Research, Inc. Issued a report that stated: 'The dream of a hydrogen economy. Is no nearer'.
It concluded that 'Capital cost. Will limit adoption to a mere 5.9 GW' by 2030, providing 'a nearly insurmountable barrier to adoption, except in niche applications'. The analysis concluded that, by 2030, PEM stationary market will reach $1 billion, while the vehicle market, including forklifts, will reach a total of $2 billion.
Other analyses cite the lack of an extensive in the U.S. As an ongoing challenge to Fuel Cell Electric Vehicle commercialization. In 2006, a study for the IEEE showed that for hydrogen produced via electrolysis of water: 'Only about 25% of the power generated from wind, water, or sun is converted to practical use.' The study further noted that 'Electricity obtained from hydrogen fuel cells appears to be four times as expensive as electricity drawn from the electrical transmission grid. Because of the high energy losses [hydrogen] cannot compete with electricity.' Furthermore, the study found: 'Natural gas reforming is not a sustainable solution'. 'The large amount of energy required to isolate hydrogen from natural compounds (water, natural gas, biomass), package the light gas by compression or liquefaction, transfer the energy carrier to the user, plus the energy lost when it is converted to useful electricity with fuel cells, leaves around 25% for practical use.'
, the author of (2005), devoted two articles in 2014 to updating his critique of the use of fuel cells in cars. He stated that FCVs still had not overcome the following issues: high cost of the vehicles, high fueling cost, and a lack of fuel-delivery infrastructure. 'It would take several miracles to overcome all of those problems simultaneously in the coming decades.' Most importantly, he said, 'FCVs aren't green' because of escaping methane during natural gas extraction and when hydrogen is produced, as 95% of it is, using the steam reforming process. He concluded that renewable energy cannot economically be used to make hydrogen for an FCV fleet 'either now or in the future.' 's analyst reached similar conclusions in 2014.
In 2015, Clean Technica listed some of the disadvantages of hydrogen fuel cell vehicles. Another Clean Technica writer concluded, 'while hydrogen may have a part to play in the world of energy storage (especially seasonal storage), it looks like a dead end when it comes to mainstream vehicles.' The world's first certified fuel cell boat (), in /Germany The world's first fuel-cell boat used an AFC system with 6.5 kW net output. Iceland has committed to converting its vast fishing fleet to use fuel cells to provide auxiliary power by 2015 and, eventually, to provide primary power in its boats. Amsterdam recently introduced its first fuel cell-powered boat that ferries people around the city's canals.
Submarines [ ] The of the German and Italian navies use fuel cells to remain submerged for weeks without the need to surface. The U212A is a non-nuclear submarine developed by German naval shipyard Howaldtswerke Deutsche Werft.
The system consists of nine PEM fuel cells, providing between 30 kW and 50 kW each. The ship is silent, giving it an advantage in the detection of other submarines. A naval paper has theorized about the possibility of a nuclear-fuel cell hybrid whereby the fuel cell is used when silent operations are required and then replenished from the Nuclear reactor (and water). Portable power systems [ ] Portable power systems that use fuel cells can be used in the leisure sector (i.e. RVs, cabins, marine), the industrial sector (i.e. Power for remote locations including gas/oil wellsites, communication towers, security, weather stations), and in the military sector.
SFC Energy is a German manufacturer of for a variety of portable power systems. Ensol Systems Inc. Is an integrator of portable power systems, using the SFC Energy DMFC.
Other applications [ ] • Providing power for or • • are a type of fuel cell system, which may include lighting, generators and other apparatus, to provide backup resources in a crisis or when regular systems fail. They find uses in a wide variety of settings from residential homes to hospitals, scientific laboratories,, • telecommunication equipment and modern naval ships. • An ( UPS) provides emergency power and, depending on the topology, provide line regulation as well to connected equipment by supplying power from a separate source when utility power is not available. Unlike a standby generator, it can provide instant protection from a momentary power interruption.
• • •, pairing the fuel cell with either an ICE or a battery. • for applications where charging may not be readily available.
• Portable charging docks for small electronics (e.g. A belt clip that charges a cell phone or ). •, laptops and tablets.
• Small heating appliances •, achieved by exhausting the oxygen and automatically maintaining oxygen exhaustion in a shipping container, containing, for example, fresh fish. •, where the amount of voltage generated by a fuel cell is used to determine the concentration of fuel (alcohol) in the sample. •, electrochemical sensor. Fueling stations [ ]. In 2013, reported that there were '10 hydrogen stations available to the public in the entire United States: one in, eight in Southern California and the one in '.
As of December 2016, there were 31 publicly accessible in the US, 28 of which were located in California. A public hydrogen refueling station in Iceland operated from 2003 to 2007.
It served three buses in the public transport net of. The station produced its own hydrogen with an electrolyzing unit. The 14 stations in Germany were planned to be expanded to 50 by 2015 through its Now GMBH. By May 2017, there were 91 hydrogen fueling stations in Japan. As of 2016, planned to build a network of hydrogen stations between the major cities, starting in 2017.
Markets and economics [ ]. Main articles: and In 2012, fuel cell industry revenues exceeded $1 billion market value worldwide, with Asian pacific countries shipping more than 3/4 of the fuel cell systems worldwide.
However, as of January 2014, no public company in the industry had yet become profitable. There were 140,000 fuel cell stacks shipped globally in 2010, up from 11,000 shipments in 2007, and from 2011 to 2012 worldwide fuel cell shipments had an annual growth rate of 85%. Expanded its manufacturing facilities in 2011. Approximately 50% of fuel cell shipments in 2010 were stationary fuel cells, up from about a third in 2009, and the four dominant producers in the Fuel Cell Industry were the United States, Germany, Japan and South Korea.
The Department of Energy Solid State Energy Conversion Alliance found that, as of January 2011, stationary fuel cells generated power at approximately $724 to $775 per kilowatt installed. In 2011, Bloom Energy, a major fuel cell supplier, said that its fuel cells generated power at 9–11 cents per kilowatt-hour, including the price of fuel, maintenance, and hardware.
Industry groups predict that there are sufficient platinum resources for future demand, and in 2007, research at suggested that platinum could be replaced by a gold- coating, which may be less susceptible to poisoning and thereby improve fuel cell lifetime. Another method would use iron and sulphur instead of platinum. This would lower the cost of a fuel cell (as the platinum in a regular fuel cell costs around US$1,500, and the same amount of iron costs only around US$1.50). The concept was being developed by a coalition of the and the. Cathodes are immune to monoxide poisoning. In 2016, 'decided to drop fuel cell-related business projects, as the outlook of the market isn't good'. Research and development [ ] • 2005: researchers used to raise the operating temperature of PEM fuel cells from below 100 °C to over 125 °C, claiming this will require less carbon-monoxide purification of the hydrogen fuel. Calendar Of Premenstrual Experiences Download.
• 2008:, used as a. • 2009: Researchers at the, in Ohio, showed that arrays of vertically grown could be used as the in fuel cells. The same year, a nickel bisdiphosphine-based catalyst for fuel cells was demonstrated.
• 2013: British firm developed a fuel cell that it said can run for 10,000 hours in simulated driving conditions. It asserted that the cost of fuel cell construction can be reduced to $40/kW (roughly $9,000 for 300 HP). • 2014: Researchers in developed a new method for regeneration of hydrogen sulfide contaminated PEFCs. They recovered 95–100% of the original performance of a hydrogen sulfide contaminated PEFC. They were successful in rejuvenating a SO 2 contaminated PEFC too. This regeneration method is applicable to multiple cell stacks. See also [ ].
A large datacenter-scale UPS being installed by electricians An uninterruptible power supply, also uninterruptible power source, UPS or battery/flywheel backup, is an electrical apparatus that provides emergency power to a load when the input power source or fails. A UPS differs from an auxiliary or or in that it will provide near-instantaneous protection from input power interruptions, by supplying energy stored in batteries,,. The on-battery runtime of most uninterruptible power sources is relatively short (only a few minutes) but sufficient to start a standby power source or properly shut down the protected equipment. A UPS is typically used to protect hardware such as,, equipment or other electrical equipment where an unexpected power disruption could cause injuries, fatalities, serious business disruption or data loss.
UPS units range in size from units designed to protect a single computer without a video monitor (around 200 rating) to large units powering entire data centers or buildings. The world's largest UPS, the 46-megawatt Battery Electric Storage System (BESS), in, powers the entire city and nearby rural communities during outages. Contents • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Common power problems [ ] The primary role of any UPS is to provide short-term power when the input power source fails.
However, most UPS units are also capable in varying degrees of correcting common utility power problems: • or sustained • Momentary or sustained • Noise, defined as a or, usually injected into the line by nearby equipment • Instability of the •, defined as a departure from the ideal expected on the line UPS units are divided into categories based on which of the above problems they address, [ – ] and some manufacturers categorize their products in accordance with the number of power-related problems they address. Technologies [ ] The three general categories of modern UPS systems are on-line, line-interactive and standby. An on-line UPS uses a 'double conversion' method of accepting AC input, to DC for passing through the (or battery strings), then inverting back to 120 V/230 V AC for powering the protected equipment. A line-interactive UPS maintains the inverter in line and redirects the battery's DC current path from the normal charging mode to supplying current when power is lost. In a standby ('off-line') system the load is powered directly by the input power and the backup power circuitry is only invoked when the utility power fails.
Most UPS below 1 kVA are of the line-interactive or standby variety which are usually less expensive. For large power units, Dynamic Uninterruptible Power Supplies (DUPS) are sometimes used.
A synchronous motor/alternator is connected on the mains via a. Energy is stored in a. When the mains power fails, an eddy-current regulation maintains the power on the load as long as the flywheel's energy is not exhausted. DUPS are sometimes combined or integrated with a diesel generator that is turned on after a brief delay, forming a (DRUPS). A UPS has been developed in recent years using hydrogen and a fuel cell as a power source, potentially providing long run times in a small space. Offline/standby [ ].
Offline/Standby UPS: The green line illustrates the flow of electric power. Typical protection time: 0–20 minutes. Capacity expansion: Usually not available The offline/standby UPS (SPS) offers only the most basic features, providing surge protection and battery backup. The protected equipment is normally connected directly to incoming utility power. When the incoming voltage falls below or rises above a predetermined level the SPS turns on its internal DC-AC inverter circuitry, which is powered from an internal storage battery. The UPS then mechanically switches the connected equipment on to its DC-AC inverter output. The switchover time can be as long as 25 milliseconds depending on the amount of time it takes the standby UPS to detect the lost utility voltage.
The UPS will be designed to power certain equipment, such as a personal computer, without any objectionable dip or to that device. Line-interactive [ ]. Line-interactive UPS: The green line illustrates the flow of electric power. Typical protection time: 5–30 minutes. Capacity expansion: several hours The line-interactive UPS is similar in operation to a standby UPS, but with the addition of a multi-tap variable-voltage.
This is a special type of that can add or subtract powered coils of wire, thereby increasing or decreasing the magnetic field and the output voltage of the transformer. This may also be performed by a which is distinct from an autotransformer, since the former may be wired to provide. This type of UPS is able to tolerate continuous undervoltage and overvoltage surges without consuming the limited reserve battery power. It instead compensates by automatically selecting different power taps on the autotransformer. Depending on the design, changing the autotransformer tap can cause a very brief output power disruption, which may cause UPSs equipped with a power-loss alarm to 'chirp' for a moment.
This has become popular even in the cheapest UPSs because it takes advantage of components already included. The main 50/60 Hz transformer used to convert between line voltage and battery voltage needs to provide two slightly different turns ratios: One to convert the battery output voltage (typically a multiple of 12 V) to line voltage, and a second one to convert the line voltage to a slightly higher battery charging voltage (such as a multiple of 14 V).
The difference between the two voltages is because charging a battery requires a delta voltage (up to 13–14 V for charging a 12 V battery). Furthermore, it is easier to do the switching on the line-voltage side of the transformer because of the lower currents on that side. To gain the buck/boost feature, all that is required is two separate switches so that the AC input can be connected to one of the two primary taps, while the load is connected to the other, thus using the main transformer's primary windings as an autotransformer. The battery can still be charged while 'bucking' an overvoltage, but while 'boosting' an undervoltage, the transformer output is too low to charge the batteries. Autotransformers can be engineered to cover a wide range of varying input voltages, but this requires more taps and increases complexity, and expense of the UPS. It is common for the autotransformer to cover a range only from about 90 V to 140 V for 120 V power, and then switch to battery if the voltage goes much higher or lower than that range.
In low-voltage conditions the UPS will use more current than normal so it may need a higher current circuit than a normal device. For example, to power a 1000-W device at 120 V, the UPS will draw 8.33 A. If a brownout occurs and the voltage drops to 100 V, the UPS will draw 10 A to compensate. This also works in reverse, so that in an overvoltage condition, the UPS will need less current.
Online/double-conversion [ ] In an online UPS, the batteries are always connected to the inverter, so that no power transfer switches are necessary. When power loss occurs, the rectifier simply drops out of the circuit and the batteries keep the power steady and unchanged.
When power is restored, the rectifier resumes carrying most of the load and begins charging the batteries, though the charging current may be limited to prevent the high-power rectifier from overheating the batteries and boiling off the electrolyte. The main advantage of an on-line UPS is its ability to provide an 'electrical firewall' between the incoming utility power and sensitive electronic equipment. The online UPS is ideal for environments where electrical isolation is necessary or for equipment that is very sensitive to power fluctuations. Although it was at one time reserved for very large installations of 10 kW or more, advances in technology have now permitted it to be available as a common consumer device, supplying 500 W or less. The initial cost of the online UPS may be higher, but its total cost of ownership is generally lower due to longer battery life. The online UPS may be necessary when the power environment is 'noisy', when utility power sags, outages and other anomalies are frequent, when protection of sensitive IT equipment loads is required, or when operation from an extended-run backup generator is necessary.
The basic technology of the online UPS is the same as in a standby or line-interactive UPS. However it typically costs much more, due to it having a much greater current AC-to-DC battery-charger/rectifier, and with the and inverter designed to run continuously with improved cooling systems. It is called a double-conversion UPS due to the rectifier directly driving the inverter, even when powered from normal AC current. Other designs [ ] Hybrid topology/double conversion on demand [ ] These hybrid Rotary UPS designs do not have official designations, although one name used by UTL is 'double conversion on demand'. This style of UPS is targeted towards high-efficiency applications while still maintaining the features and protection level offered by double conversion. A hybrid (double conversion on demand) UPS operates as an off-line/standby UPS when power conditions are within a certain preset window.
This allows the UPS to achieve very high efficiency ratings. When the power conditions fluctuate outside of the predefined windows, the UPS switches to online/double-conversion operation. In double-conversion mode the UPS can adjust for voltage variations without having to use battery power, can filter out line noise and control frequency. Ferroresonant [ ] Ferroresonant units operate in the same way as a standby UPS unit; however, they are online with the exception that a, is used to filter the output.
This transformer is designed to hold energy long enough to cover the time between switching from line power to battery power and effectively eliminates the transfer time. Many ferroresonant UPSs are 82–88% efficient (AC/DC-AC) and offer excellent isolation. The transformer has three windings, one for ordinary mains power, the second for rectified battery power, and the third for output AC power to the load.
This once was the dominant type of UPS and is limited to around the 150 kVA range. These units are still mainly used in some industrial settings (oil and gas, petrochemical, chemical, utility, and heavy industry markets) due to the robust nature of the UPS. Many ferroresonant UPSs utilizing controlled ferro technology may not interact with power-factor-correcting equipment. [ ] DC power [ ] A UPS designed for powering DC equipment is very similar to an online UPS, except that it does not need an output inverter.
Cod4 Promod Bots Download. Also, if the UPS's battery voltage is matched with the voltage the device needs, the device's power supply will not be needed either. Since one or more power conversion steps are eliminated, this increases efficiency and run time. Many systems used in telecommunications use an ' 48 V DC power, because it has less restrictive safety regulations, such as being installed in conduit and junction boxes. DC has typically been the dominant power source for telecommunications, and AC has typically been the dominant source for computers and servers.
There has been much experimentation with 48 V DC power for computer servers, in the hope of reducing the likelihood of failure and the cost of equipment. However, to supply the same amount of power, the current would be higher than an equivalent 115 V or 230 V circuit; greater current requires larger conductors, or more energy lost as heat. A laptop computer is a classic example of a PC with a DC UPS built in.
High voltage DC (380 V) is finding use in some data center applications, and allows for small power conductors, but is subject to the more complex electrical code rules for safe containment of high voltages. Rotary [ ] A rotary UPS uses the inertia of a high-mass spinning () to provide short-term ride-through in the event of power loss. The flywheel also acts as a buffer against power spikes and sags, since such short-term power events are not able to appreciably affect the rotational speed of the high-mass flywheel. It is also one of the oldest designs, predating vacuum tubes and integrated circuits. It can be considered to be on line since it spins continuously under normal conditions. However, unlike a battery-based UPS, flywheel-based UPS systems typically provide 10 to 20 seconds of protection before the flywheel has slowed and power output stops. It is traditionally used in conjunction with standby generators, providing backup power only for the brief period of time the engine needs to start running and stabilize its output.
The rotary UPS is generally reserved for applications needing more than 10,000 W of protection, to justify the expense and benefit from the advantages rotary UPS systems bring. A larger flywheel or multiple flywheels operating in parallel will increase the reserve running time or capacity. Because the flywheels are a mechanical power source, it is not necessary to use an electric motor or generator as an intermediary between it and a diesel engine designed to provide emergency power. By using a transmission gearbox, the rotational inertia of the flywheel can be used to directly start up a diesel engine, and once running, the diesel engine can be used to directly spin the flywheel. Multiple flywheels can likewise be connected in parallel through mechanical, without the need for separate motors and generators for each flywheel.
They are normally designed to provide very high current output compared to a purely electronic UPS, and are better able to provide inrush current for inductive loads such as motor startup or compressor loads, as well as medical MRI and equipment. It is also able to tolerate short-circuit conditions up to 17 times larger than an electronic UPS, permitting one device to blow a fuse and fail while other devices still continue to be powered from the rotary UPS. Its life cycle is usually far greater than a purely electronic UPS, up to 30 years or more. But they do require periodic downtime for mechanical maintenance, such as replacement. In larger systems redundancy of the system ensures the availability of processes during this maintenance. Battery-based designs do not require downtime if the batteries can be, which is usually the case for larger units.
Newer rotary units use technologies such as and air-evacuated enclosures to increase standby efficiency and reduce maintenance to very low levels. Typically, the high-mass flywheel is used in conjunction with a system. These units can be configured as: • A motor driving a mechanically connected generator, • A combined and generator wound in alternating slots of a single rotor and stator, • A hybrid rotary UPS, designed similar to an online UPS, except that it uses the flywheel in place of batteries. The rectifier drives a motor to spin the flywheel, while a generator uses the flywheel to power the inverter. In case No. 3 the motor generator can be synchronous/synchronous or induction/synchronous. The motor side of the unit in case Nos. 2 and 3 can be driven directly by an AC power source (typically when in inverter bypass), a 6-step double-conversion motor drive, or a 6-pulse inverter.
Case No. 1 uses an integrated flywheel as a short-term energy source instead of batteries to allow time for external, electrically coupled gensets to start and be brought online. Case Nos. 2 and 3 can use batteries or a free-standing electrically coupled flywheel as the short-term energy source. Form factors [ ] UPS systems come in several different forms and sizes. However, the two most common forms are tower and rack-mount. Tower model [ ] Tower models stand upright on the ground or on a desk/shelf, and are typically used in network workstations or desktop computer applications. Rack-mount model [ ] Rack-mount models can be mounted in standard 19' rack enclosures and can require anywhere from 1U to 12U (rack space).
They are typically used in server and networking applications. Applications [ ] N+1 [ ] In large business environments where reliability is of great importance, a single huge UPS can also be a single point of failure that can disrupt many other systems. To provide greater reliability, multiple smaller UPS modules and batteries can be integrated together to provide power protection equivalent to one very large UPS.
'N+1' means that if the load can be supplied by N modules, the installation will contain N+1 modules. In this way, failure of one module will not impact system operation.
Multiple redundancy [ ] Many computer servers offer the option of redundant power supplies, so that in the event of one power supply failing, one or more other power supplies are able to power the load. This is a critical point – each power supply must be able to power the entire server by itself. Redundancy is further enhanced by plugging each power supply into a different circuit (i.e. To a different ).
Redundant protection can be extended further yet by connecting each power supply to its own UPS. This provides double protection from both a power supply failure and a UPS failure, so that continued operation is assured. This configuration is also referred to as 1+1 or 2N redundancy. If the budget does not allow for two identical UPS units then it is common practice to plug one power supply into and the other into the UPS. Outdoor use [ ] When a UPS system is placed outdoors, it should have some specific features that guarantee that it can tolerate weather without any effects on performance. Factors such as temperature,, rain, and snow among others should be considered by the manufacturer when designing an outdoor UPS system.
Ranges for outdoor UPS systems could be around −40 °C to +55. Outdoor UPS systems can either be pole, ground (pedestal), or host mounted. Outdoor environment could mean extreme cold, in which case the outdoor UPS system should include a battery heater mat, or extreme heat, in which case the outdoor UPS system should include a fan system or an air conditioning system. Internal view of a solar inverter. Note the many large capacitors (blue cylinders), used to store energy briefly and improve the output waveform. A solar inverter, or PV inverter, or solar converter, converts the variable (DC) output of a (PV) into a (AC) that can be fed into a commercial electrical or used by a local, electrical network. It is a critical –component in a, allowing the use of ordinary AC-powered equipment.
Solar inverters have special functions adapted for use with photovoltaic arrays, including and anti- protection. Difficulties faced with generator use [ ] Power factor [ ]. See also: A problem in the combination of a double-conversion UPS and a generator is the voltage distortion created by the UPS. The input of a double-conversion UPS is essentially a big rectifier. The current drawn by the UPS is non-sinusoidal. This can cause the voltage from the AC mains or a generator to also become non-sinusoidal. The voltage distortion then can cause problems in all electrical equipment connected to that power source, including the UPS itself.
It will also cause more power to be lost in the wiring supplying power to the UPS due to the spikes in current flow. This level of 'noise' is measured as a percentage of ' (THD I). Classic UPS rectifiers have a THD I level of around 25%–30%. To reduce voltage distortion, this requires heavier mains wiring or generators more than twice as large as the UPS.
There are several solutions to reduce the THD I in a double-conversion UPS: Passive power-factor correction [ ]. Main article: An alternative solution is an active filter. Through the use of such a device, THD I can drop to 5% over the full power range. The newest technology in double-conversion UPS units is a rectifier that does not use classic rectifier components (thyristors and diodes) but uses high-frequency components instead. A double-conversion UPS with an rectifier and inductor can have a THD I as small as 2%.
This completely eliminates the need to oversize the generator (and transformers), without additional filters, investment cost, losses, or space. Communication [ ]. This section needs expansion.
You can help. (December 2009) (PM) requires • The UPS to report its status to the computer it powers via a communications link such as a, and, GSM/ or • A subsystem in the that processes the reports and generates notifications, PM events, or commands an ordered shut down. Some UPS manufacturers publish their communication protocols, but other manufacturers (such as ) use. The basic computer-to-UPS control methods are intended for one-to-one signaling from a single source to a single target.
For example, a single UPS may connect to a single computer to provide status information about the UPS, and allow the computer to control the UPS. Similarly, the USB protocol is also intended to connect a single computer to multiple peripheral devices. In some situations it is useful for a single large UPS to be able to communicate with several protected devices. For traditional serial or USB control, a signal replication device may be used, which for example allows one UPS to connect to five computers using serial or USB connections.
However, the splitting is typically only one direction from UPS to the devices to provide status information. Return control signals may only be permitted from one of the protected systems to the UPS. As Ethernet has increased in common use since the 1990s, control signals are now commonly sent between a single UPS and multiple computers using standard Ethernet data communication methods such as. The status and control information is typically encrypted so that for example an outside hacker can not gain control of the UPS and command it to shut down. Distribution of UPS status and control data requires that all intermediary devices such as Ethernet switches or serial multiplexers be powered by one or more UPS systems, in order for the UPS alerts to reach the target systems during a. To avoid the dependency on Ethernet infrastructure, the UPSs can be connected directly to main control server by using GSM/GPRS channel also.
The SMS or GPRS data packets sent from UPSs trigger software to shut down the PCs to reduce the load. Batteries [ ]. Battery cabinet The run-time for a battery-operated UPS depends on the type and size of batteries and rate of discharge, and the efficiency of the inverter. The total capacity of a is a function of the rate at which it is discharged, which is described as. Manufacturers supply run-time rating in minutes for packaged UPS systems. Larger systems (such as for data centers) require detailed calculation of the load, inverter efficiency, and battery characteristics to ensure the required endurance is attained. Common battery characteristics and load testing [ ] When a lead–acid battery is charged or discharged, this initially affects only the reacting chemicals, which are at the interface between the electrodes and the electrolyte.
With time, the charge stored in the chemicals at the interface, often called 'interface charge', spreads by of these chemicals throughout the volume of the active material. If a battery has been completely discharged (e.g.
The car lights were left on overnight) and next is given a fast charge for only a few minutes, then during the short charging time it develops only a charge near the interface. The battery voltage may rise to be close to the charger voltage so that the charging current decreases significantly. After a few hours this interface charge will spread to the volume of the electrode and electrolyte, leading to an interface charge so low that it may be insufficient to start a car. Due to the interface charge, brief UPS self-test functions lasting only a few seconds may not accurately reflect the true runtime capacity of a UPS, and instead an extended recalibration or rundown test that deeply discharges the battery is needed. The deep discharge testing is itself damaging to batteries due to the chemicals in the discharged battery starting to into highly stable molecular shapes that will not re-dissolve when the battery is recharged, permanently reducing charge capacity. In lead acid batteries this is known as but also affects other types such as and.
Therefore, it is commonly recommended that rundown tests be performed infrequently, such as every six months to a year. Testing of strings of batteries/cells [ ] Multi- commercial UPS systems with large and easily accessible battery banks are capable of isolating and testing individual cells within a battery string, which consists of either combined-cell battery units (such as 12-V lead acid batteries) or individual chemical cells wired in series. Isolating a single cell and installing a jumper in place of it allows the one battery to be discharge-tested, while the rest of the battery string remains charged and available to provide protection. It is also possible to measure the electrical characteristics of individual cells in a battery string, using intermediate sensor wires that are installed at every cell-to-cell junction, and monitored both individually and collectively. Battery strings may also be wired as series-parallel, for example two sets of 20 cells.
In such a situation it is also necessary to monitor current flow between parallel strings, as current may circulate between the strings to balance out the effects of weak cells, dead cells with high resistance, or shorted cells. For example, stronger strings can discharge through weaker strings until voltage imbalances are equalized, and this must be factored into the individual inter-cell measurements within each string. Series-parallel battery interactions [ ] Battery strings wired in can develop unusual failure modes due to interactions between the multiple parallel strings.
Defective batteries in one string can adversely affect the operation and lifespan of good or new batteries in other strings. These issues also apply to other situations where series-parallel strings are used, not just in UPS systems but also in applications.
Consider a series-parallel battery arrangement with all good cells, and one becomes shorted or dead: • The failed cell will reduce the maximum developed voltage for the entire series string it is within. • Other series strings wired in parallel with the degraded string will now discharge through the degraded string until their voltage matches the voltage of the degraded string, potentially overcharging and leading to boiling and outgassing from the remaining good cells in the degraded string. These parallel strings can now never be fully recharged, as the increased voltage will bleed off through the string containing the failed battery. • Charging systems may attempt to gauge battery string capacity by measuring overall voltage. Due to the overall string voltage depletion due to the dead cells, the charging system may detect this as a state of discharge, and will continuously attempt to charge the series-parallel strings, which leads to continuous overcharging and damage to all the cells in the degraded series string containing the damaged battery.
• If are used, all cells in the formerly good parallel strings will begin to sulfate due to the inability for them to be fully recharged, resulting in the storage capacity of these cells being permanently damaged, even if the damaged cell in the one degraded string is eventually discovered and replaced with a new one. The only way to prevent these subtle series-parallel string interactions is by not using parallel strings at all and using separate charge controllers and inverters for individual series strings. Series new/old battery interactions [ ] Even just a single string of batteries wired in series can have adverse interactions if new batteries are mixed with old batteries. Older batteries tend to have reduced storage capacity, and so will both discharge faster than new batteries and also charge to their maximum capacity more rapidly than new batteries. As a mixed string of new and old batteries is depleted, the string voltage will drop, and when the old batteries are exhausted the new batteries still have charge available.
The newer cells may continue to discharge through the rest of the string, but due to the low voltage this energy flow may not be useful, and may be wasted in the old cells as resistance heating. For cells that are supposed to operate within a specific discharge window, new cells with more capacity may cause the old cells in the series string to continue to discharge beyond the safe bottom limit of the discharge window, damaging the old cells. When recharged, the old cells recharge more rapidly, leading to a rapid rise of voltage to near the fully charged state, but before the new cells with more capacity have fully recharged.
The charge controller detects the high voltage of a nearly fully charged string and reduces current flow. The new cells with more capacity now charge very slowly, so slowly that the chemicals may begin to crystallize before reaching the fully charged state, reducing new cell capacity over several charge/discharge cycles until their capacity more closely matches the old cells in the series string. For such reasons, some industrial UPS management systems recommend periodic replacement of entire battery arrays potentially using hundreds of expensive batteries, due to these damaging interactions between new batteries and old batteries, within and across series and parallel strings. Standards [ ] • EN 62040-1:2008 Uninterruptible power systems (UPS) – Part 1: General and safety requirements for UPS • EN 62040-2:2006 Uninterruptible power systems (UPS) – Part 2: Electromagnetic compatibility (EMC) requirements • EN 62040-3:2011 Uninterruptible power systems (UPS) – Part 3: Method of specifying the performance and test requirements • EN 62040-4:2013 Uninterruptible power systems (UPS) - Part 4: Environmental aspects - Requirements and reporting See also [ ] • • • • • • • • (SMPS) • References [ ]. Energy Information Administration (EIA). Retrieved July 23, 2012. • E-book on choosing a UPS topology based on application type (PDF).
(2002), A new international UPS classification by IEC 62040-3,: • Detailed explanation of UPS topologies (PDF). November 2000. Archived from on October 4, 2013. Archived from (PDF) on December 4, 2014.
• ^ [ ] • My Ton (Ecos Consulting), Brian Fortenbery (EPRI), William Tschudi (LNBL) (January 2007). Lawrence Berkeley National Laboratory. Archived from (PDF) on 2008-08-20.
CS1 maint: Uses authors parameter () • Active Power. • Tripp Lite: UPS Buying Guide, • Detailed explanation of optimized N+1 configurations (PDF). • Detailed explanation of UPS redundancy options (PDF). • Refer to safety standard IEC 60950-22 or a local derivative according to location e.g. EN 60950-22 (Europe); UL 60950-22 (USA) • Raymond, Eric Steven.. The Linux Documentation Project, 2003–2007. Multi-XS is an active RS232 data switch, designed to handle serial communications of one UPS with up to 5 / 10 computers • APC AP9207 Share-UPS, User Manual, pp.
6–7, Port 1 is called the Advanced port because it supplies smart signaling, which provides the advanced capabilities available to a server running PowerChute plus software. The Advanced port provides full access to the Computer Interface port of the UPS. Ports 2–8 on the rear panel of Share-UPS are called Basic ports because they supply simple UPS signaling for On Battery and Low Battery conditions in the UPS. Archived from (PDF) on April 24, 2012. Retrieved November 14, 2011. • An example of an Ethernet UPS controller: • APC Application Note #67 (PDF). Archived from (PDF) on April 24, 2012.
Retrieved November 14, 2011. PowerStream Technologies. Retrieved 2010-04-26. • Saslow, Wayne M. Electricity, Magnetism, and Light.
Toronto: Thomson Learning. Curtis (2011).. Hordeski (2005).. The Fairmont Press, Inc.. • Leonardo Energy..
Retrieved August 1, 2012. [ ] • APC Inc.. The Data Center Journal.
[ ] • and, page 18, showing sensor wires for each cell/battery on a battery string, and also note that the current transducer sensors to detect cross-string series-parallel current recirculation. •, Battery and Energy Technologies, Cell Balancing, Woodbank Communications Ltd, Chester, UK. • 2013-04-06 at the., Battery Asset Management: VRLA ageing characteristics, Bart Cotton, founder and CEO, Data Power Monitoring Corporation, Batteries International, Jan 2005 External links [ ] Wikimedia Commons has media related to.
• Scott Siddens (February 2007),, Plant Engineering, archived from on 2009-11-09 • Cottuli, Carol (2011), (PDF), Schneider Electric, White Paper 92 rev. 2, retrieved April 7, 2012 • Rasmussen, Neil (2011), (PDF), Schneider Electric, White Paper 1 rev. 7, retrieved April 7, 2012 • VanDee, Dawn (March 1, 1999),, EC&M, Penton Business Media, retrieved April 7, 2012 •. Eaton Corporation. Retrieved 2014-01-08. •, to support Power Devices. Which also highlights various aspects of such devices.
•, archived from on 2015-05-30.